To describe a spin system it's necessary to state which nucleus is coupled with which. When two equivalent nuclei have identical relations with the same identical partners, they are “magnetically equivalent”. Only in this case it's possible to define them as a group and not individually.
Two nuclei are magnetically equivalent when they have:
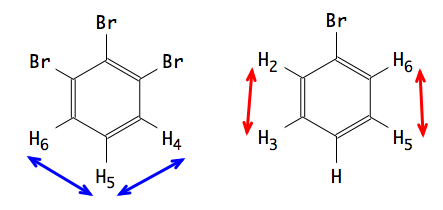
At left, H4 and H6 are magnetically equivalent, because they are both coupled with H5 and with no other.
At right, H2 and H6 are related by symmetry, therefore they have the same chemical shift and the same coupling constants.
Their partners, however, are different: H2 is coupled with H3, while H6 is not (or not with the same intensity).
In conclusion. H2 and H6 are NOT magnetically equivalent and must be declared separately.
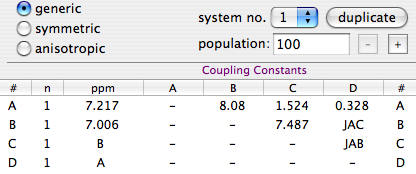
This table, which refers to ortho-dichloro-benzene, provides an illuminating demonstration of what the concept of magnetic equivalence is all about. Nuclei A and D have identical chemical shifts and identical coupling constants. One may therefore be tempted to put them into the same row. When it's time to specify the first J he is soon in trouble. JDB=1.524, while JAB=8.08, so whatever he writes it's wrong in at least one case.
Groups of magnetically equivalent nuclei are frequently encountered. A common example is represented by the 3 hydrogens of a methyl residue. The two members of a methylenic pair, on the contrary, rarely are magnetically equivalent, and often they don't even share the same chemical shift!